Honey’s natural sugars are dehydrated to prevent fermentation, with added enzymes to modify and transform their chemical composition and pH. Invertases and digestive acids hydrolyze sucrose to give the monosaccharides glucose and fructose. The invertase is one of these enzymes synthesized by the body of the insect.
Honey bees transform saccharides into honey by a process of regurgitation, a number of times, until it is partially digested. The bees do the regurgitation and digestion as a group. After the last regurgitation, the aqueous solution is still high in water, so the process continues by evaporation of much of the water and enzymatic transformation.
Honey is produced by bees as a food source. In cold weather or when fresh food sources are scarce, bees use their stored honey as their source of energy By contriving for bee swarms to nest in artificial hives, people have been able to semidomesticate the insects, and harvest excess honey. In the hive (or in a wild nest), there are three types of bees:
- a single female queen bee
- a seasonally variable number of male drone bees to fertilize new queens
- some 20,000 to 40,000 female worker bees.
The worker bees raise larvae and collect the nectar that will become honey in the hive. Leaving the hive, they collect sugar-rich flower nectar and return.
In the hive, the bees use their “honey stomachs” to ingest and regurgitate the nectar a number of times until it is partially digested. Invertase synthesized by the bees and digestive acids hydrolyze sucrose to give the same mixture of glucose and fructose. The bees work together as a group with the regurgitation and digestion until the product reaches a desired quality. It is then stored in honeycomb cells. After the final regurgitation, the honeycomb is left unsealed. However, the nectar is still high in both water content and natural yeasts, which, unchecked, would cause the sugars in the nectar to ferment. The process continues as bees inside the hive fan their wings, creating a strong draft across the honeycomb, which enhances evaporation of much of the water from the nectar. This reduction in water content raises the sugar concentration and prevents fermentation. Ripe honey, as removed from the hive by a beekeeper, has a long shelf life, and will not ferment if properly sealed.
Physical and chemical properties
Crystallized honey. The inset shows a close-up of the honey, showing the individual glucose grains in the fructose mixture.
The physical properties of honey vary, depending on water content, the type of flora used to produce it (pasturage), temperature, and the proportion of the specific sugars it contains. Fresh honey is a supersaturated liquid, containing more sugar than the water can typically dissolve at ambient temperatures. At room temperature, honey is a supercooled liquid, in which the glucose will precipitate into solid granules. This forms a semisolid solution of precipitated glucose crystals in a solution of fructose and other ingredients.
Phase transitions
The melting point of crystallized honey is between 40 and 50 °C (104 and 122 °F), depending on its composition. Below this temperature, honey can be either in a metastable state, meaning that it will not crystallize until a seed crystal is added, or, more often, it is in a “labile” state, being saturated with enough sugars to crystallize spontaneously. The rate of crystallization is affected by many factors, but the primary factor is the ratio of the main sugars: fructose to glucose. Honeys that are supersaturated with a very high percentage of glucose, such as brassica honey, will crystallize almost immediately after harvesting, while honeys with a low percentage of glucose, such as chestnut or tupelo honey, do not crystallize. Some types of honey may produce very large but few crystals, while others will produce many small crystals.
Crystallization is also affected by water content, because a high percentage of water will inhibit crystallization, as will a high dextrin content. Temperature also affects the rate of crystallization, with the fastest growth occuring between 13 and 17 °C (55 and 63 °F). Crystal nuclei (seeds) tend to form more readily if the honey is disturbed, by stirring, shaking or agitatiing, rather than if left at rest. However, the nucleation of microscopic seed-crystals is greatest between 5 and 8 °C (41 and 46 °F). Therefore, larger but fewer crystals tend to form at higher temperatures, while smaller but more-numerous crystals usually form at lower temperatures. Below 5 °C, the honey will not crystallize and, thus, the original texture and flavor can be preserved indefinitely
Since honey normally exists below its melting point, it is a supercooled liquid. At very low temperatures, honey will not freeze solid. Instead, as the temperatures become colder, the viscosity of honey increases. Like most viscous liquids, the honey will become thick and sluggish with decreasing temperature. At −20 °C (−4 °F), honey may appear or even feel solid, but it will continue to flow at very slow rates. Honey has a glass transition between -42 and -51 °C (-44 and -60 °F). Below this temperature, honey enters a glassy state and will become an amorphous solid (noncrystalline).
Viscosity
The viscosity of honey is affected greatly by both temperature and water content. The higher the humidity, the easier honey will flow. Above its melting point, however, water has little effect on viscosity. Aside from water content, the composition of honey also has little effect on viscosity, with the exception of a few types. At 25 °C (77 °F), honey with 14% humidity will generally have a viscosity of around 400 poise, while a honey containing 20% humidity will have a viscosity of around 20 poise. Viscosity increase due to temperature occurs very slowly at first. A honey containing 16% humidity, at 70 °C (158 °F), will have a viscosity of around 2 poise, while at 30 °C (86 °F), the viscosity will be around 70 poise. As cooling progresses, honey will become more viscous at an increasingly rapid rate, reaching 600 poise around 14 °C (57 °F). However, while honey is very viscous, it has rather low surface tension.
A few types of honey have unusual viscous properties. Honey from heather or manuka display thixotropic properties. These types of honey enter a gel-like state when motionless, but then liquify when stirred.
Electrical and optical properties
Since honey contains electrolytes, in the form of acids and minerals, it exhibits varying degrees of electrical conductivity. Measurements of the electrical conductivity are used to determine the quality of honey in terms of ash content.
The effect honey has on light is useful for determining the type and quality. Variations in the water content alter the refractive index of honey. Water content can easily be measured with a refractometer. Typically, the refractive index for honey will range from 1.504 at 13% humidity, to 1.474 at 25%. Honey also has an effect on polarized light, in that it will rotate the polarization plane. The fructose will give a negative rotation, while the glucose will give a positive one. The overall rotation can be used to measure the ratio of the mixture.
Hygroscopy and fermentation
Honey has the ability to absorb moisture directly from the air, a phenomenon called hygroscopy. The amount of water the honey will absorb is dependent on the relative humidity of the air. Because honey contains yeast, this hygroscopic nature requires that honey be stored in sealed containers to prevent fermentation, which usually begins if the honey’s humidity rises much above 25%. Honey will tend to absorb more water in this manner than the individual sugars would allow on their own, which may be due to other ingredients it contains.
Fermentation of honey will usually occur after crystallization because, without the glucose, the liquid portion of the honey primarily consists of a concentrated mixture of the fructose, acids, and water, providing the yeast with enough of an increase in the water percentage for growth. Honey that is to be stored at room temperature for long periods of time is often pasteurized, to kill any yeast, by heating it above 70 °C (158 °F).
Thermal characteristics
Like all sugar compounds, honey will caramelize if heated too much, becoming darker in color and eventually burning. However, honey contains fructose, which caramelizes at lower temperatures than the glucose.[20] Honey also contains acids, which act as catalysts, decreasing the caramelization temperature even more. Of these acids, the amino acids, which occur in very small amounts, play an important role in the darkening of honey. The amino acids form darkened compounds called melanoidins, during a Maillard reaction. The temperature at which caramelization begins varies, depending on the composition, but is typically between 70 °C (158 °F) and 110 °C (230 °F). The Maillard reaction will occur slowly at room temperature, taking from a few to several months to show visible darkening, but will speed-up dramatically with increasing temperatures. However, the reaction can also be slowed by storing the honey at colder temperatures.
Unlike many other liquids, honey has very poor thermal conductivity, taking a long time to reach thermal equilibrium. Melting crystallized honey can easily result in localized caramelization if the heat source is too hot, or if it is not evenly distributed. However, honey will take substantially longer to liquify when just above the melting point than it will at elevated temperatures. Melting 20 kilograms of crystallized honey, at 40 °C (104 °F), can take up to 24 hours, while 50 kilograms may take twice as long. These times can be cut nearly in half by heating at 50 °C (122 °F). However, many of the minor substances in honey can be affected greatly by heating, changing the flavor, aroma, or other properties, so heating is usually done at the lowest temperature possible for the shortest amount of time.
Acid content
The average pH of honey is 3.9, but can range from 3.4 to 6.1. Honey contains many kinds of acids, both organic and amino. However, the different types and their amounts vary considerably, depending on the type of honey. These acids may be aromatic or aliphatic (non-aromatic). The aliphatic acids contribute greatly to the flavor of honey by interacting with the flavors of other ingredients. Gluconic acid, for instance, is a flavor enhancer. The aromatic acids, such as malic acid, come mostly from the flowers, adding to the aroma and taste of the honey.
Honey can contain up to 18 of the 20 amino acids. However, amino acid content is almost negligible in honey, accounting for only 0.05–0.1% of the composition. The main amino acid is proline. Amino acids are derived almost solely from the bodies of the bees.
Organic acids comprise most of the acids in honey, accounting for 0.17–1.17% of the mixture. Gluconic acid is the most prevalent. Gluconic acid is formed by the actions of an enzyme called glucose oxidase. Other organic acids are minor, consisting of formic, acetic, butyric, citric, lactic, malic, pyroglutamic, propionic, valeric, capronic, palmitic, and succinic, among many others.
In history, culture, and folklore
Honey use and production has a long and varied history. In many cultures, honey has associations that go beyond its use as a food. Honey is frequently used as a talisman and symbol of sweetness.
Ancient times
Honey collection is an ancient activity. Humans apparently began hunting for honey at least 8,000 years ago, as evidenced by a cave painting in Valencia, Spain. The painting is a Mesolithic rock painting, showing two honey-hunters collecting honey and honeycomb from a wild bee nest. The figures are depicted carrying baskets or gourds, and using a ladder or series of ropes to reach the wild nest.
The Greater Honeyguide guides humans to wild bee hives and this behavior may have evolved with early hominids.
So far, the oldest remains of honey have been found in Georgia. Archaeologists have found honey remains on the inner surface of clay vessels unearthed an ancient tomb, dating back to some 4,700–5,500 years ago. In ancient Georgia, honey was packed for people’s journeys into the afterlife. And more than one type, too – along for the trip were linden, berry, and a meadow-flower variety.
In ancient Egypt, honey was used to sweeten cakes and biscuits, and was used in many other dishes. Ancient Egyptian and Middle Eastern peoples also used honey for embalming the dead. The fertility god of Egypt, Min, was offered honey.
Pliny the Elder devotes considerable space in his book Naturalis Historia to the bee and honey, and its many uses. In the absence of sugar, Honey was an integral sweetening ingredient in Roman recipes, and references to its use in food can be found in the work of many Roman authors including Athenaeus, Cato and Bassus. Some of these are collected in the book Roman cookery.
The art of beekeeping in ancient China has existed since time immemorial and appears to be untraceable to its origin. In the book “Golden Rules of Business Success” written by Fan Li (or Tao Zhu Gong) during the Spring and Autumn Period, there are some parts mentioning the art of beekeeping and the importance of the quality of the wooden box for bee keeping that can affect the quality of its honey.
Honey was also cultivated in ancient Mesoamerica. The Maya used honey from the stingless bee for culinary purposes, and continue to do so today. The Maya also regard the bee as sacred (see Mayan stingless bees of Central America).
Some cultures believed honey had many practical health uses. It was used as an ointment for rashes and burns, and to help soothe sore throats when no other practices were available.
Religious significance
In Hinduism, honey (Madhu) is one of the five elixirs of immortality (Panchamrita). In temples, honey is poured over the deities in a ritual called Madhu abhisheka. The Vedas and other ancient literature mention the use of honey as a great medicinal and health food.
In Jewish tradition, honey is a symbol for the new year, Rosh Hashanah. At the traditional meal for that holiday, apple slices are dipped in honey and eaten to bring a sweet new year. Some Rosh Hashanah greetings show honey and an apple, symbolizing the feast. In some congregations, small straws of honey are given out to usher in the new year.
The Hebrew Bible contains many references to honey. In the Book of Judges, Samson found a swarm of bees and honey in the carcass of a lion (14:8). The Book of Exodus famously describes the Promised Land as a “land flowing with milk and honey” (33:3). However, the claim has been advanced that the original Hebrew (דבש devash) actually refers to the sweet syrup produced from the juice of dates. Pure honey is considered kosher even though it is produced by a flying insect, a nonkosher creature; other products of nonkosher animals are not kosher.
In Buddhism, honey plays an important role in the festival of Madhu Purnima, celebrated in India and Bangladesh. The day commemorates Buddha’s making peace among his disciples by retreating into the wilderness. The legend has it that while he was there, a monkey brought him honey to eat. On Madhu Purnima, Buddhists remember this act by giving honey to monks. The monkey’s gift is frequently depicted in Buddhist art.
In the Christian New Testament, Matthew 3:4, John the Baptist is said to have lived for a long period of time in the wilderness on a diet consisting of locusts and wild honey.
In Islam, there is an entire Surah in the Qur’an called al-Nahl (the Honey Bee). According to hadith, Prophet Muhammad strongly recommended honey for healing purposes. The Qur’an promotes honey as a nutritious and healthy food. Below is the English translation of those specific verses.
And your Lord inspired the bee(s), saying: “Take your habitations in the mountains and in the trees and in what they erect. (68) Then, eat of all fruits, and follow the ways of your Lord made easy (for you).” There comes forth from their bellies, a drink of varying colour wherein is healing for mankind. Verily, in this is indeed a sign for people who think.[38]
In western culture
Jars of honey with honeycomb
The word “honey”, along with variations like “honey bun” and the abbreviation “hon”, has become a term of endearment in most of the English-speaking world. In some places it is used for loved ones; in others, such as the Southern United States, it is used when addressing casual acquaintances or even strangers.
Collecting honey
Honey is collected from wild bee colonies, or from domesticated beehives. Wild bee nests are sometimes located by following a honeyguide bird.
Collecting honey is typically achieved by using smoke from a bee smoker to pacify the bees; this causes the bees to attempt to save the resources of the hive from a possible forest fire, and makes them far less aggressive. The honeycomb is removed from the hive and the honey is extracted from that, often using a honey extractor. The honey is then filtered.
Modern uses
As a food and in cooking
The main uses of honey are in cooking, baking, as a spread on bread, and as an addition to various beverages, such as tea, and as a sweetener in some commercial beverages. According to the The National Honey Board (a USDA-overseen organization), “honey stipulates a pure product that does not allow for the addition of any other substance…this includes, but is not limited to, water or other sweeteners”. Honey barbecue and honey mustard are common and popular sauce flavors.
Honey is the main ingredient in the alcoholic beverage mead, which is also known as “honey wine” or “honey beer”. Historically, the ferment for mead was honey’s naturally occurring yeast. Honey is also used as an adjunct in some beers.
Honey wine, or mead, is typically (modern era) made with a honey and water mixture with a pack of yeast added for fermentation. Primary fermentation usually takes 40 days, after which the must needs to be racked into a secondary fermentation vessel and left to sit about 35–40 more days. If done properly, fermentation will be finished by this point (though if a sparkling mead is desired, fermentation can be restarted after bottling by the addition of a small amount of sugar), but most meads require aging for 6–9 months or more in order to be palatable.
Nutrition
|
Honey |
|
|
Nutritional value per 100 g (3.5 oz) |
|
|
Energy |
1,272 kJ (304 kcal) |
|
Carbohydrates |
82.4 g |
|
– Sugars |
82.12 g |
|
– Dietary fiber |
0.2 g |
|
Fat |
0 g |
|
Protein |
0.3 g |
|
Water |
17.10 g |
| Riboflavin (vit. B2) | 0.038 mg (3%) |
| Niacin (vit. B3) | 0.121 mg (1%) |
| Pantothenic acid (B5) | 0.068 mg (1%) |
| Vitamin B6 | 0.024 mg (2%) |
| Folate (vit. B9) | 2 μg (1%) |
| Vitamin C | 0.5 mg (1%) |
| Calcium | 6 mg (1%) |
| Iron | 0.42 mg (3%) |
| Magnesium | 2 mg (1%) |
| Phosphorus | 4 mg (1%) |
| Potassium | 52 mg (1%) |
| Sodium | 4 mg (0%) |
| Zinc | 0.22 mg (2%) |
|
Shown is for 100 g, roughly 5 tbsp. |
|
Honey is a mixture of sugars and other compounds. With respect to carbohydrates, honey is mainly fructose (about 38.5%) and glucose (about 31.0%), making it similar to the synthetically produced inverted sugar syrup, which is approximately 48% fructose, 47% glucose, and 5% sucrose. Honey’s remaining carbohydrates include maltose, sucrose, and other complex carbohydrates.[1] As with all nutritive sweeteners, honey is mostly sugars and contains only trace amounts of vitamins or minerals. Honey also contains tiny amounts of several compounds thought to function as antioxidants, including chrysin, pinobanksin, vitamin C, catalase, and pinocembrin. The specific composition of any batch of honey depends on the flowers available to the bees that produced the honey.
Typical honey analysis:
- Fructose: 38.2%
- Glucose: 31.3%
- Maltose: 7.1%
- Sucrose: 1.3%
- Water: 17.2%
- Higher sugars: 1.5%
- Ash: 0.2%
- Other/undetermined: 3.2%
Its glycemic index ranges from 31 to 78, depending on the variety.
Honey has a density of about 1.36 kilograms per litre (36% denser than water).
Isotope ratio mass spectrometry can be used to detect addition of corn syrup or sugar cane sugars by the carbon isotopic signature. Addition of sugars originating from corn or sugar cane (C4 plants, unlike the plants used by bees, which are predominantly C3 plants) skews the isotopic ratio of sugars present in honey, but does not influence the isotopic ratio of proteins; in an unadulterated honey, the carbon isotopic ratios of sugars and proteins should match. As low as 7% level of addition can be detected.
Classification
Honey is classified by its floral source, and there are also divisions according to the packaging and processing used. There are also regional honeys. Honey is also graded on its color and optical density by USDA standards, graded on a scale called the Pfund scale, which ranges from 0 for “water white” honey to more than 114 for “dark amber” honey.
Floral source
Generally, honey is classified by the floral source of the nectar from which it was made. Honeys can be from specific types of flower nectars or can be blended after collection. The pollen in honey is traceable to floral source and therefore region of origin. The rheological & mellisopalynological properties of honey can be used to identify the major plant nectar source used in its production.
Blended
Most commercially available honey is blended, meaning it is a mixture of two or more honeys differing in floral source, color, flavor, density or geographic origin.
Polyfloral
Polyfloral honey, also known as wildflower honey, is derived from the nectar of many types of flowers.[
The taste may vary from year to year, and the aroma and the flavor can be more or less intense, depending on which bloomings are prevalent.
Monofloral
Monofloral honey is made primarily from the nectar of one type of flower. Different monofloral honeys have a distinctive flavor and color because of differences between their principal nectar sources. To produce monofloral honey, beekeepers keep beehives in an area where the bees have access to only one type of flower. In practice, because of the difficulties in containing bees, a small proportion of any honey will be from additional nectar from other flower types. Typical examples of North American monofloral honeys are clover, orange blossom, blueberry, sage, tupelo, buckwheat, fireweed, and sourwood. Some typical European examples include thyme, thistle, heather, acacia, dandelion, sunflower, honeysuckle, and varieties from lime and chestnut trees. In North Africa (e.g. Egypt) examples include clover, cotton, and citrus (mainly orange blossoms).
Honeydew honey
Instead of taking nectar, bees can take honeydew, the sweet secretions of aphids or other plant sap-sucking insects. Honeydew honey is very dark brown in color, with a rich fragrance of stewed fruit or fig jam, and is not as sweet as nectar honeys. Germany’s Black Forest is a well known source of honeydew-based honeys, as well as some regions in Bulgaria, Tara (mountain) in Serbia and Northern California in the United States. In Greece, pine honey (a type of honeydew honey) constitutes 60–65% of the annual honey production. Honeydew honey is popular in some areas, but in other areas beekeepers have difficulty selling the stronger flavored product.
The production of honeydew honey has some complications and dangers. The honey has a much larger proportion of indigestibles than light floral honeys, thus causing dysentery to the bees, resulting in the death of colonies in areas with cold winters. Good beekeeping management requires the removal of honeydew prior to winter in colder areas. Bees collecting this resource also have to be fed protein supplements, as honeydew lacks the protein-rich pollen accompaniment gathered from flowers.
Classification by packaging and processing
Generally, honey is bottled in its familiar liquid form. However, honey is sold in other forms, and can be subjected to a variety of processing methods.
Honeycomb
Crystallized honey is honey in which some of the glucose content has spontaneously crystallized from solution as the monohydrate. Also called “granulated honey” or “candied honey.” Honey that has crystallized (or comercially purchased crystallized) can be returned to a liquid state by warming.
Pasteurized honey is honey that has been heated in a pasteurization process which requires temperatures of 161 °F (72 °C) or higher. Pasteurization destroys yeast cells. It also liquefies any microcrystals in the honey, which delays the onset of visible crystallization. However, excessive heat exposure also results in product deterioration, as it increases the level of hydroxymethylfurfural (HMF) and reduces enzyme (e.g. diastase) activity. Heat also affects appearance (darkens the natural honey color), taste, and fragrance.
Raw honey is honey as it exists in the beehive or as obtained by extraction, settling or straining, without adding heat (although some honey that has been “minimally processed” is often labeled as raw honey). Raw honey contains some pollen and may contain small particles of wax. Local raw honey is sought after by allergy sufferers as the pollen impurities are thought to lessen the sensitivity to hay fever.
Strained honey has been passed through a mesh material to remove particulate material (pieces of wax, propolis, other defects) without removing pollen, minerals or enzymes.
Filtered honey is honey of any type that has been filtered to the extent that all or most of the fine particles, pollen grains, air bubbles, or other materials normally found in suspension, have been removed. The process typically heats honey to 150–170 °F (66–77 °C) to more easily pass through the filter. Filtered honey is very clear and will not crystallize as quickly, making it preferred by the supermarket trade.
Ultrasonicated honey has been processed by ultrasonication, a nonthermal processing alternative for honey. When honey is exposed to ultrasonication, most of the yeast cells are destroyed. Those cells that survive sonication generally lose their ability to grow, which reduces the rate of honey fermentation substantially. Ultrasonication also eliminates existing crystals and inhibits further crystallization in honey. Ultrasonically aided liquefaction can work at substantially lower temperatures of approximately 95 °F (35 °C) and can reduce liquefaction time to less than 30 seconds.
Creamed honey, also called whipped honey, spun honey, churned honey, candied honey, honey fondant, and set honey (in the UK), has been processed to control crystallization. Creamed honey contains a large number of small crystals, which prevent the formation of larger crystals that can occur in unprocessed honey. The processing also produces a honey with a smooth, spreadable consistency.
Dried honey has the moisture extracted from liquid honey to create completely solid, nonsticky granules. This process may or may not include the use of drying and anticaking agents. Dried honey is commonly used to garnish desserts.
Comb honey is honey still in the honeybees’ wax comb. It traditionally is collected by using standard wooden frames in honey supers. The frames are collected and the comb is cut out in chunks before packaging. As an alternative to this labor intensive method, plastic rings or cartridges can be used that do not require manual cutting of the comb, and speed packaging. Comb honey harvested in the traditional manner is also referred to as “cut-comb honey”. In India, honey is harvested from forests in bee’s natural habitat. It is said that honey will be consumed by the bees on the new moon day, so it is cultivated the day before.
Chunk honey is packed in widemouth containers consisting of one or more pieces of comb honey immersed in extracted liquid honey.
Preservation
Sealed frame of honey
Because of its unique composition and chemical properties, honey is suitable for long-term storage, and is easily assimilated even after long preservation. Honey, and objects immersed in honey, have been preserved for decades and even centuries. The key to preservation is limiting access to humidity. In its cured state, honey has a sufficiently high sugar content to inhibit fermentation. If exposed to moist air, its hydrophilic properties will pull moisture into the honey, eventually diluting it to the point that fermentation can begin. Honey sealed in honeycomb cells by the bees is considered by many to be the ideal form for preservation.
Honey should also be protected from oxidation and temperature degradation. It generally should not be preserved in metal containers because the acids in the honey may promote oxidation of the vessel. Traditionally, honey was stored in ceramic or wooden containers; however, glass and plastic are now the favored materials. Honey stored in wooden containers may be discolored or take on flavors imparted from the vessel. Likewise, honey stored uncovered near other foods may absorb other smells.
Excessive heat can have detrimental effects on the nutritional value of honey. Heating up to 37 °C (99 °F) causes loss of nearly 200 components, some of which are antibacterial. Heating up to 40 °C (104 °F) destroys invertase, an important enzyme. At 50 °C (122 °F), the honey sugars caramelize. Generally, any large temperature fluctuation causes decay.Regardless of preservation, honey may crystallize over time. Crystallization does not affect the flavor, quality or nutritional content of the honey, though it does affect color and texture. The rate is a function of storage temperature, availability of “seed” crystals and the specific mix of sugars and trace compounds in the honey. Tupelo and acacia honeys, for example, are exceptionally slow to crystallize, while goldenrod will often crystallize still in the comb. Most honeys crystallize fastest between about 50 and 70 °F (10 and 21 °C). The crystals can be dissolved by heating the honey.
Distinguishing honey
Honey grading
In the US, honey grading is performed voluntarily (USDA does offer inspection and grading “as on-line (in-plant) or lot inspection…upon application, on a fee-for-service basis.”) based upon USDA standards. Honey is graded based upon a number of factors, including water content, flavor and aroma, absence of defects and clarity. Honey is also classified by color though it is not a factor in the grading scale. The honey grade scale is:
|
Grade |
Water content |
Flavor and aroma |
Absence of defects |
Clarity |
|
A |
< 18.6% | Good—has a good, normal flavor and aroma for the predominant floral source and is free from caramelization, smoke, fermentation, chemicals and other odor causes | Practically free—practically no defects that affect appearance or edibility | Clear—may contain air bubbles that do not materially affect the appearance; may contain a trace of pollen grains or other finely divided particles of suspended material that do not affect appearance |
|
B |
< 18.6% | Reasonably good—practically free from caramelization; free from smoke, fermentation, chemicals, and other causes | Reasonably free—do not materially affect appearance or edibility | Reasonably clear—may contain air bubbles, pollen grains, or other finely divided particles of suspended material that do not materially affect appearance |
|
C |
< 20.0% | Fairly good—reasonably free from caramelization; free from smoke, fermentation, chemicals, and other causes | Fairly free—do not seriously affect the appearance or edibility | Fairly clear—may contain air bubbles, pollen grains, or other finely divided particles of suspended material that do not seriously affect appearance |
|
Substandard |
> 20.0% | Fails Grade C | Fails Grade C | Fails Grade C |
Other countries may have differing standards on the grading of honey. India, for example, certifies honey grades based on additional factors, such as the Fiehe’s test, and other empirical measurements.
Indicators of quality
High-quality honey can be distinguished by fragrance, taste, and consistency. Ripe, freshly collected, high-quality honey at 20 °C (68 °F) should flow from a knife in a straight stream, without breaking into separate drops. After falling down, the honey should form a bead. The honey, when poured, should form small, temporary layers that disappear fairly quickly, indicating high viscosity. If not, it indicates excessive water content (over 20%)[ of the product. Honey with excessive water content is not suitable for long-term preservation.
In jars, fresh honey should appear as a pure, consistent fluid, and should not set in layers. Within a few weeks to a few months of extraction, many varieties of honey crystallize into a cream-colored solid. Some varieties of honey, including tupelo, acacia, and sage, crystallize less regularly. Honey may be heated during bottling at temperatures of 40–49°C (104–120°F) to delay or inhibit crystallization. Overheating is indicated by change in enzyme levels, for instance, diastase activity, which can be determined with the Schade or the Phadebas methods. A fluffy film on the surface of the honey (like a white foam), or marble-colored or white-spotted crystallization on a containers sides, is formed by air bubbles trapped during the bottling process.
A 2008 Italian study determined nuclear magnetic resonance spectroscopy can be used to distinguish between different honey types, and can be used to pinpoint the area where it was produced. Researchers were able to identify differences in acacia and polyfloral honeys by the differing proportions of fructose and sucrose, as well as differing levels of aromatic amino acids phenylalanine and tyrosine. This ability allows greater ease of selecting compatible stocks.
In medicine
Historically, honey has been used by humans to treat a variety of ailments, from gastric disturbances to ulcers, wounds and burns, through ingestion or topical application, but only recently have the antiseptic and antibacterial properties of honey been chemically explained. Different honeys have different properties, which was known since ancient times. Much scientific research has been done, with emphasis of late on fighting infections in wounds. The antibacterial mechanisms known to date are H2O2, methylglyoxal(MGO), bee defensin-1, the osmotic effect and the pH.[
In Ayurveda, a 4000-year-old treatise on medicine originating from India, honey is considered to positively affect all three primitive material imbalances of the body. “Vaatalam guru sheetam cha raktapittakaphapaham| Sandhatru cchedanam ruksham kashayam madhuram madhu|| “It has sweetness with added astringent as end taste. It is heavy, dry and cold. Its effect on doshas (imbalances) is that it aggravates vata (air / moving forces), scrapes kapha (mucus / holding forces) and normalizes pitta (catabolic fire) and rakta (blood). It promotes the healing process.” Some wound gels which contain antibacterial raw honey and have regulatory approval are now available to help treat drug-resistant strains of bacteria (MRSA). One New Zealand researcher says a particular type of honey (manuka honey) may be useful in treating MRSA infections.)
As an antimicrobial agent honey is useful in treating a variety of ailments. Antibacterial properties of honey are the result of the low water activity causing osmosis, chelation of free iron, its slow release of hydrogen peroxide, high acidity, and the antibacterial activity of methylglyoxal.
Honey appears to be effective in killing drug-resistant biofilms which are implicated in chronic rhinosinusitis.
Osmotic effect
Honey has an osmotic effect.Honey is primarily a saturated mixture of two monosaccharides, with a low water activity; most of the water molecules are associated with the sugars and few remain available for microorganisms, so it is a poor environment for their growth. If water is mixed with honey, it loses its low water activity, and therefore no longer possesses this antimicrobial property.
Hydrogen peroxide
Hydrogen peroxide is formed in a slow-release manner by the enzyme glucose oxidase present in honey. It becomes active only when honey is diluted, requires oxygen to be available for the reaction (thus it may not work under wound dressings, in wound cavities or in the gut), is active only when the acidity of honey is neutralized by body fluids, can be destroyed by the protein-digesting enzymes present in wound fluids, and is destroyed when honey is exposed to heat and light. Honey chelates and deactivates free iron, which would otherwise catalyze the formation of oxygen free radicals from hydrogen peroxide, leading to inflammation. Also, the antioxidant constituents in honey help clean up oxygen free radicals present.
C6H12O6 + H2O + O2 → C6H12O7 + H2O2 (glucose oxidase reaction)
When honey is used topically (as, for example, a wound dressing), hydrogen peroxide is produced by dilution of the honey with body fluids. As a result, hydrogen peroxide is released slowly and acts as an antiseptic.
Use for diabetic ulcers
Topical honey has been used successfully in a comprehensive treatment of diabetic ulcers when the patient cannot use topical antibiotics.
Acidity
The pH of honey is commonly between 3.2 and 4.5. This relatively acidic pH level prevents the growth of many bacteria.
Methylglyoxal
The nonperoxide antibiotic activity is due to methylglyoxal (MGO) and bee defensin-1. Most honeys contain very low levels of MGO, but manuka honey contains very high levels. The presence of the synergist in manuka honey more than doubles MGO antibacterial activity.
Nutraceutical effects
Antioxidants in honey have even been associated with reducing the damage done to the colon in colitis in a study involving administering honey enemas to rats. Such claims are consistent with its use in many traditions of folk medicine.
Use for sore throats and coughs
Honey has also been used for centuries as a treatment for sore throats and coughs and, according to recent research, may be an effective soothing agent for coughs.
Other medical applications
Unfiltered, pasteurized honey is widely believed to alleviate allergies, though neither commercially filtered nor raw honey was shown to be more effective than placebo in a controlled study of 36 participants with ocular allergies.] Nearly 1 in 3 of the volunteers dropped out of the study because they couldn’t tolerate eating one tablespoon of honey every day due to the overly sweet taste.[ The official conclusion: “This study does not confirm the widely held belief that honey relieves the symptoms of allergic rhinoconjunctivitis.” A more recent study has shown pollen collected by bees to exert an antiallergenic effect, mediated by an inhibition of IgE immunoglobulin binding to mast cells. This inhibited mast cell degranulation and thus reduced allergic reaction. The risk of experiencing anaphylaxis as an immune system reaction may outweigh any potential allergy relief.
A review in the Cochrane Library suggests honey could reduce the time it takes for a mild burn to heal — up to four days sooner in some cases. The review included 19 studies with 2,554 participants. Although the honey treatment healed mild burns faster than traditional dressings did, the author recommends viewing the findings with caution, since a single research centre performed all of the burn studies.
Health hazards
Botulism
Because of the natural presence of botulinum endospores in honey, children under one year of age should not be given honey. The more-developed digestive system of older children and adults generally destroys the spores. Infants, however, can contract botulism from honey. Medical grade honey can be treated with gamma radiation to reduce the risk of botulinum spores being present. Gamma radiation evidently does not affect honey’s antibacterial activity, whether or not the particular honey’s antibacterial activity is dependent upon peroxide generation.
Infantile botulism shows geographical variation. In the UK, only six cases have been reported between 1976 and 2006, yet the U.S. has much higher rates: 1.9 per 100,000 live births, 47.2% of which are in California. While the risk honey poses to infant health is small, it is recommended not to take the risk.
Toxic honey
Main article: Bees and toxic chemicals#Toxic honey
Honey produced from flowers of oleanders, rhododendrons, mountain laurels,[disambiguation needed] sheep laurel, and azaleas may cause honey intoxication. Symptoms include dizziness, weakness, excessive perspiration, nausea, and vomiting. Less commonly, low blood pressure, shock, heart rhythm irregularities, and convulsions may occur, with rare cases resulting in death. Honey intoxication is more likely when using “natural” unprocessed honey and honey from farmers who may have a small number of hives. Commercial processing, with pooling of honey from numerous sources, claims it dilutes any toxins but these findings are not verifiable.[
New Zealand
Toxic honey may also result when bees are proximate to tutu bushes (Coriaria arborea) and the vine hopper insect (Scolypopa australis). Both are found throughout New Zealand. Bees gather honeydew produced by the vine hopper insects feeding on the tutu plant. This introduces the poison tutin into honey. Only a few areas in New Zealand (Coromandel Peninsula, Eastern Bay of Plenty and the Marlborough Sound) frequently produce toxic honey. Symptoms of tutin poisoning include vomiting, delirium, giddiness, increased excitability, stupor, coma, and violent convulsions. To reduce the risk of tutin poisoning, humans should not eat honey taken from feral hives in the risk areas of New Zealand. Since December 2001, New Zealand beekeepers have been required to reduce the risk of producing toxic honey by closely monitoring tutu, vine hopper, and foraging conditions within 3 km of their apiary.
Honey-producing and consuming countries
Honey-producing countries
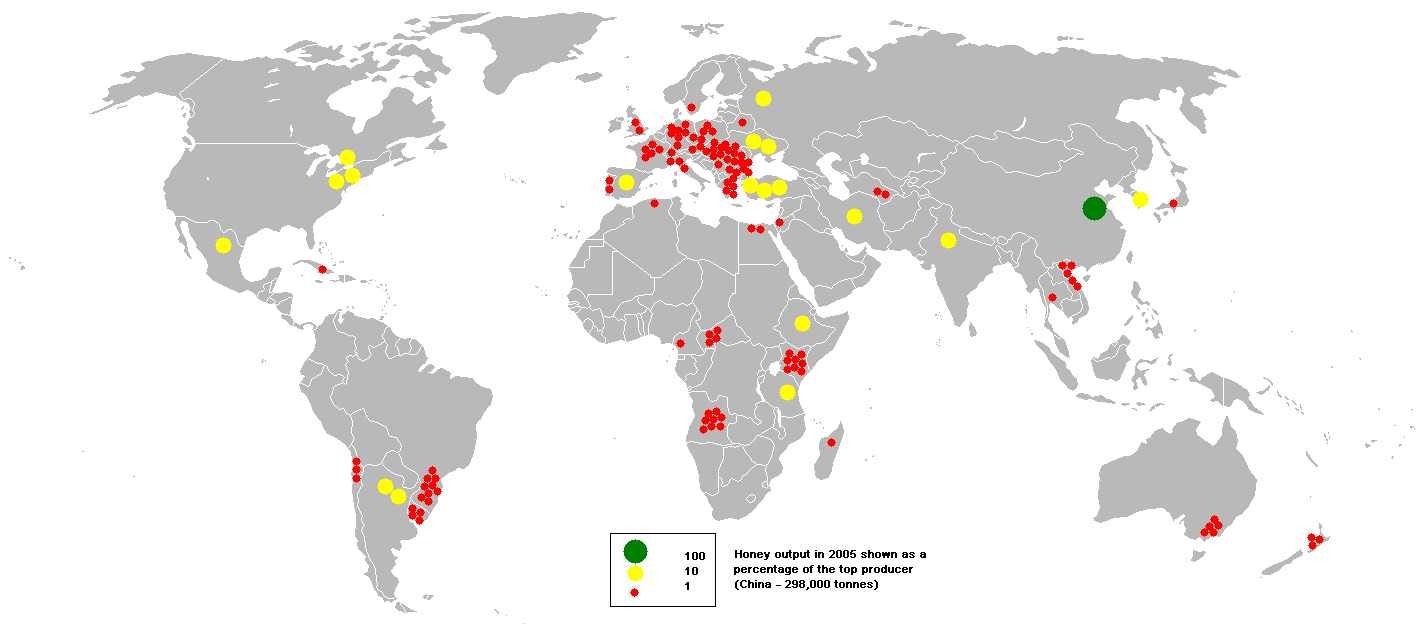
In 2012, China, Turkey, and the United States were the top producers of natural honey.
Significant regional producers of honey include Ukraine (ranked fourth worldwide) and Argentina (ranked fifth worldwide).
Mexico is also an important producer of honey, providing more than 4% of the world’s supply.Much of this (about one-third) comes from the Yucatán Peninsula. Honey production began there when the Apis mellifera and the A. mellifera ligustica were introduced there early in the 20th century. Most of Mexico’s Yucatán producers are small, family operations who use original traditional techniques, moving hives to take advantage of the various tropical and subtropical flowers.
Honey is also one of the gourmet products of the French island of Corsica. Corsican honey is certified as to its origin (Appellation d’origine contrôlée) just as are French wines, like Champagne.
Honey consumption per capita per year exceeds one kilogram in some countries like Austria, Germany and Switzerland.